Compounds and Mixtures
Grade 8 Science Worksheets
What is a Compound?
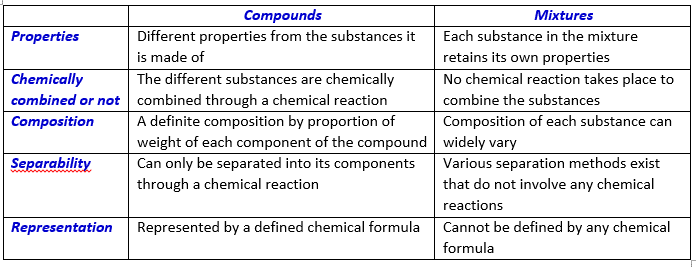
A Compound is a substance that contains more than one kind of atom. It has a definite composition and can be described by a chemical formula. Millions of compounds occur in nature and several can even be artificially produced.
Compounds are formed when the atoms of two or more elements chemically react in defined proportions of weight. The simplest example of a compound is water, a liquid, which consists of two atoms of hydrogen and one atom of oxygen. Its chemical formula is H2O.
Carbon and hydrogen are two elements whose atoms combine in numerous proportions to create millions of compounds – methane (CH4), ethane (C2H6), propane (C3H8), butane (C4H10), to name just a few, are all gaseous compounds.
Every day substances like table salt (sodium chloride, NaCl), baking soda (NaHCO3) and even ice which is nothing but ‘solid water’, are examples of solid compounds.
What is a Mixture?
A Mixture is formed of two or more elements and/or compounds which are physically mixed together. The molecules of the individual components do not combine chemically to form new molecules in the mixture. For example, if you dissolve salt in water, both compounds, you get salt water, but the salt and water molecules continue to retain their original properties.
The striking difference between a compound and a mixture is that a compound can be broken down into its individual components (atoms or molecules) only through a chemical reaction. The components of a mixture, however, can be separated through various physical means without the need for any chemical reaction.
Water, which is a compound, can only be broken down into its individual components of hydrogen and oxygen through a chemical reaction called Electrolysis.
A salt and water mixture can, however, simply be separated by evaporation of the water, leaving salt crystals behind. Or, if you mixed sand and water, you could simply filter the water through a sieve to separate the two compounds.
Schedule a Free session to clear worksheet doubts
No credit card required, no obligation to purchase.
Just schedule a FREE Sessions to meet a tutor and get help on any topic you want!
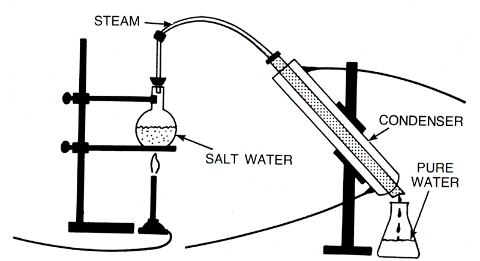
Separating Mixtures
There are many techniques to separate the components of a mixture. A method to remove light-weight husk from heavier grain particles is simply by blowing the husk away with wind, which is called Winnowing.
For heavier particles such as stones or dead insects mixed in pulses, rice or wheat grains, we use a method known as Handpicking a dry mixture – we simply pick those components out by hand to make the mixture purer or cleaner. Plucking fruit off trees is another example. Handpicking works best when the mixture contains components of different sizes or color or shape.
Evaporation technique may be used for soluble solids – salts dissolved in water, for example. Heating the solution of salt water will cause the water to evaporate, leaving salt crystals behind.
In a Simple Distillation process, the water vapor in the above example is separately collected and re-condensed to form water again.
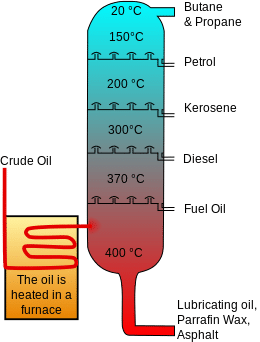
A more complicated process of Fractional Distillation is used to separate two or more liquids which have dissolved into each other, taking advantage of the different boiling points of the various liquids. This process is also useful for separating the various components of crude oil to isolate fuels such as diesel, petrol, kerosene and natural gas.
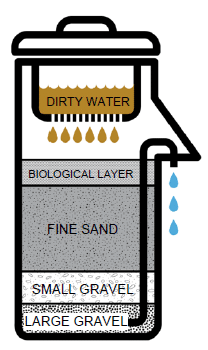
Insoluble solids, such as sand particles in water, are removed through Filtration. The water in our taps comes through a process of filtration of water from the river.
Learn more about Compounds and Mixtures and other important topics with 8th Grade Science Tutoring at eTutorWorld. Our expert science tutors break down the topics through interactive one-to-one sessions. We also offer the advantage of customized lesson plans, flexible schedules and convenience of learning from home.
eTutorWorld Understands Math Tutoring | Online Math Worksheets are Important Tools
Understanding graphs, charts, and opinion polls in a newspaper, for calculating house and car payments, and for choosing a long-distance telephone service are impossible without strong math skills …and the only way to develop strong math skills is by constant practice.
‘Practice makes a man perfect’ holds true for no other field better than for math. A middle or high school student must set aside a minimum of an hour for math every day. Other than textbooks, worksheets help you revise and understand concepts better.
Our expert tutors prepare online maths worksheets that are age and grade-appropriate. Grade-wise math worksheets for Elementary Math, Arithmetic, Pre-Algebra, Algebra, Geometry, Trigonometry, Statistics, Pre-Calculus and Calculus can be solved to improve math skills, to get ahead or to even catch up.
You may download these FREE online math worksheets in the PDF format, and then print and email us their solutions for a free evaluation and analysis by eTutorworld’smath expert tutors.
You may solve these worksheets by yourself or with your peers while studying together.
The Answer Key at the end of each worksheet allows for a self-evaluation.
Personalized Online Tutoring
eTutorWorld offers affordable one-on-one live tutoring over the web for Grades K-12, Test Prep help for Standardized tests like SCAT, CogAT, MAP, SSAT, SAT, ACT, ISEE and AP. You may schedule online tutoring lessons at your personal scheduled times, all with a Money-Back Guarantee. The first one-on-one online tutoring lesson is always FREE, no purchase obligation, no credit card required.
For answers/solutions to any question or to learn concepts, take a FREE CLASS.
No credit card required, no obligation to purchase.
Just book a free class to meet a tutor and get help on any topic you want!
Magnetism is used to separate mixtures of two solids of which one has magnetic properties. It is useful to separate magnetic metals such as iron, nickel or cobalt, that are mixed with non-metals such as gold, silver or aluminum.
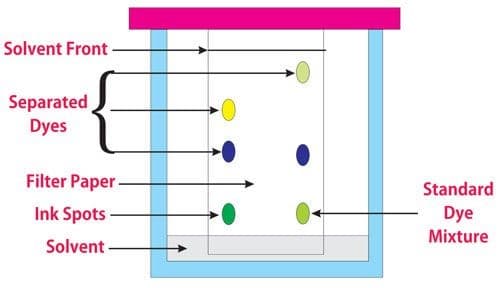
A technique known as Chromatography is used to separate a mixture through an Adsorbent Material which attracts the mixed substances to varying extents causing them to separate from one another. An adsorbent material allows dissolved solids to adhere to its surface. Adsorbent materials such as silica gel or alumina or activated carbon are used in this technique.
The food industry uses Thin Layer or Paper Chromatography to identify chemical coloring agents in foods and inks. Other chromatography techniques include Liquid Column and Gas Chromatography.
Compounds vs Mixtures

Check Point
State whether true or false –
- Compounds may be solid, liquid or gas.
- Mixtures may be solid, liquid or gas.
- Components of a compound are combined chemically and have a defined chemical formula.
- Mixtures require a chemical process to isolate its individual components.
- Gas Chromatography and winnowing are chemical processes.
Answer Key
- True
- True
- True
- False
- False
Schedule a Free session to clear worksheet doubts
No credit card required, no obligation to purchase.
Just schedule a FREE Sessions to meet a tutor and get help on any topic you want!
Pricing for Online Tutoring
| Tutoring Package | Validity | Grade (1-12), College |
|---|---|---|
| 5 sessions | 1 Month | $139 |
| 1 session | 1 Month | $28 |
| 10 sessions | 3 months | $269 |
| 15 sessions | 3 months | $399 |
| 20 sessions | 4 months | $499 |
| 50 sessions | 6 months | $1189 |
| 100 sessions | 12 months | $2249 |
8th Grade Free Worksheets
- The Universe
- Heredity
- Evolutionary Theory
- Structure of the atom
- Ethical Practices
- Unveiling the mystery behind the physical universe
- Components of the universe
- Celestial phenomena
- The tilt of Earth’s axis
- The causes of high and low tides
- Earth Systems
- Rocks and Fossils
- Weather and Climate
- Basics of chemical reactions
- Types of Chemical reactions – Endothermic, exothermic, oxidation, reduction reactions
- Catalysts and enzymes
- Compounds and mixtures
- Acids, Bases and pH Indicators
Images Credit:
https://upload.wikimedia.org/wikipedia/commons/thumb/1/13/Distillation_3_%28PSF%29.png/1200px-Distillation_3_%28PSF%29.png
https://upload.wikimedia.org/wikipedia/commons/0/0e/Coffee_beans_being_sorted_and_pulped.jpg
https://upload.wikimedia.org/wikipedia/commons/d/da/Ohorizons_Concrete_BioSand_Filter.png
https://upload.wikimedia.org/wikipedia/commons/thumb/6/6e/Crude_Oil_Distillation-en.svg/390px-Crude_Oil_Distillation-en.svg.png
IN THE NEWS

Our mission is to provide high quality online tutoring services, using state of the art Internet technology, to school students worldwide.
Online test prep and practice
SCAT
SSAT
ISEE
PSAT
SAT
ACT
AP Exam
Science Tutoring
Physics Tutoring
Chemistry Tutoring
Biology Tutoring
Math Tutoring
Pre-Algebra Tutoring
Algebra Tutoring
Pre Calculus Tutoring
Calculus Tutoring
Geometry Tutoring
Trigonometry Tutoring
Statistics Tutoring
Quick links
Free Worksheets
Fact sheet
Sales Partner Opportunities
Parents
Passive Fundraising
Virtual Fundraising
Our Expert Tutors
Safe and Secure Tutoring
Interactive Online Tutoring
After School Tutoring
Elementary School Tutoring
Middle School Tutoring
High School Tutoring
Home Work Help
Math Tutors New York City
Press
©2022 eTutorWorld Terms of use Privacy Policy Site by Little Red Bird
©2022 eTutorWorld
Terms of use
Privacy Policy
Site by Little Red Bird






