Chemical Reactions Worksheets (Explanation and Examples)
Grade 8 Science Worksheets
A chemical reaction is a process in which one or more substances, known as reactants, undergo a chemical change to form different substances, known as products. During a chemical reaction, the atoms in the reactants rearrange their connections and form new bonds to create the products.
Already familiar with the concept? Test your knowledge with our Free Chemical Reactions Worksheets!
Chemical Reactions Worksheets and More..
A chemical reaction is when one or more substances chemically convert to one or more other substances. The substance or substances that start the chemical reaction are known as Reactants. The substance or substances that are the result of the chemical reaction are known as Products. The physical and chemical properties of the products are different from those of the reactants.
An essential aspect of a chemical reaction is that the atoms of the reactants are chemically rearranged to create the new product or products. The bonds between atoms in the reactants are broken and the atoms are bonded in a new way to create the resulting products.
The destruction of bonds consumes energy while the creation of new bonds releases energy. However, there is no change in the total amount of energy as a result of a chemical reaction. Energy may change form during a chemical reaction but energy can neither be created nor destroyed. This is the Law of Conservation of Energy. Most chemical reactions involve the release of energy in the form of heat. Some reactions, however, involve the absorption of energy.
eTutorWorld Understands Math Tutoring | Online Math Worksheets are Important Tools
Understanding graphs, charts, and opinion polls in a newspaper, for calculating house and car payments, and for choosing a long-distance telephone service are impossible without strong math skills …and the only way to develop strong math skills is by constant practice.
‘Practice makes a man perfect’ holds true for no other field better than for math. A middle or high school student must set aside a minimum of an hour for math every day. Other than textbooks, worksheets help you revise and understand concepts better.
Our expert tutors prepare online maths worksheets that are age and grade-appropriate. Grade-wise math worksheets for Elementary Math, Arithmetic, Pre-Algebra, Algebra, Geometry, Trigonometry, Statistics, Pre-Calculus and Calculus can be solved to improve math skills, to get ahead or to even catch up.
You may download these FREE online math worksheets in the PDF format, and then print and email us their solutions for a free evaluation and analysis by eTutorworld’smath expert tutors.
You may solve these worksheets by yourself or with your peers while studying together.
The Answer Key at the end of each worksheet allows for a self-evaluation.
Personalized Online Tutoring
eTutorWorld offers affordable one-on-one live tutoring over the web for Grades K-12, Test Prep help for Standardized tests like SCAT, CogAT, MAP, SSAT, SAT, ACT, ISEE and AP. You may schedule online tutoring lessons at your personal scheduled times, all with a Money-Back Guarantee. The first one-on-one online tutoring lesson is always FREE, no purchase obligation, no credit card required.
For answers/solutions to any question or to learn concepts, take a FREE CLASS.
No credit card required, no obligation to purchase.
Just book a free class to meet a tutor and get help on any topic you want!
Chemical reactions are occurring all around us. Some everyday examples are –
- The rusting of iron, where iron combines with oxygen to form a red-colored oxide
- Combustion process when you strike a match
- Photosynthesis process in plants wherein carbon dioxide and water convert into glucose and oxygen
- When vinegar and baking soda combine to form a bubbling mixture
- Batteries which convert stored chemical energy into electrical energy
Chemical Equations
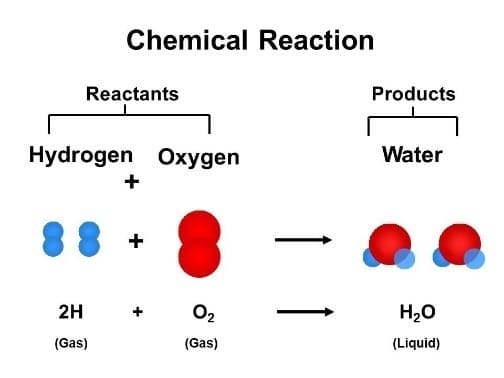
Chemical equations are a shorthand representation of what occurs in chemical reactions. Equations are written with chemical symbols and numbers to explain the reactants and products involved in a reaction. The reactants are shown on the left side of the equation while the products are shown on the right. An arrow in the middle means “yields” or “produces”.
For example –
2H2(g)+ O2(g)→ 2H2O(l)
This equation explains that two molecules of hydrogen gas(g)react with one molecule of oxygen gas(g) to yield two molecules of a compound called water (represented as H2O), which is liquid (l).
A chemical reaction between ammonia gas (NH3) and oxygen gas which yields Nitric Acid (HNO3) and water is represented as –
NH3(g)+ 2O2(g)→ HNO3(aq)+ H2O(l)
This equation explains that one molecule of ammonia gas(g)reacts with two molecules of oxygen gas(g)to yield one molecule of nitric acid, which is an aqueous solution (aq), and one molecule of water, which is liquid (l). (An aqueous solution simply means that it is ‘dissolved in the water’).
Balancing Chemical Equations
Chemical equations need to be balanced. This follows the law of conservation of matter wherein matter can neither be created nor destroyed as a result of a chemical reaction. In other words, the number of atoms of each element in an equation must remain the same on either side of that equation.
In the equation to create water, if we simply wrote:
H2(g)+ O2(g)→ H2O (l) and counted the atoms on either side, we’d get:
Left side: 2 atoms of hydrogen (represented as H2) and 2 atoms of oxygen (represented as O2)
Right side: 2 atoms of hydrogen (represented as H2) but only one atom of oxygen (O).
This is an unbalanced equation!
To correctly balance equations we make use of Coefficients. A number written before an element is known as a coefficient.
Since oxygen atoms are unbalanced, we add “2” before the water molecule H2O to correctly show 2 atoms of oxygen on the right.
(Note 1: An equation cannot be balanced by adding or removing a subscript – for example, adding a subscript to oxygen on the right side to show “H2O2” might balance the equation but it is chemically incorrect. This is because H2O2 is a completely different substance – hydrogen peroxide – compared to water – H2O).
(Note 2: The coefficient “2” before H2O applies to both H2 and to O. The coefficient is multiplied by the number of atoms in the subscript of the element to give us the total number of atoms for that element. This coefficient therefore gives us 4 atoms of hydrogen and 2 atoms of oxygen on the right side of the equation).
Thus:H2(g)+ O2(g)→ 2H2O (l) helps balance the oxygen atoms on either side but leaves the hydrogen atoms unbalanced. The hydrogen count of atoms is 2 on the left side and 4 on the right. The equation is still unbalanced!
To completely balance the equation, we need yet another coefficient “2” on the left side before the hydrogen element.
Thus: 2H2(g)+ O2(g)→ 2H2O (l) which now correctly gives –
Left side: 4 atoms of hydrogen and 2 atoms of oxygen
Right side: 4 atoms of hydrogen and 2 atoms of oxygen
In molecular terms, 2 molecules of hydrogen combine with one molecule of oxygen to give two molecules of water.
The equation is now balanced!
Stoichiometry
Balancing a chemical equation is the first step to performing what chemists call Stoichiometry – using the balanced equation to determine amounts of reactants and products. It provides chemists, who have to deal with large quantities of actual substances, a way to understand the atomic world of atoms and molecules in terms of the real world of mass of substances.
To do this, chemists convert units of substances to Moles. Chemists have defined the mole of any substance in equivalent terms to the mass of atoms in 12 grams of Carbon-12. In other words, 1 mole of carbon weighs exactly 12 grams. Using comparative atomic masses between Carbon and other elements, the weight of other elements is determined. Hence, 1 mole of hydrogen weighs 1 gram, 1 mole of oxygen weighs 16 grams. And so on.
In stoichiometric terms the chemical equation to produce water, 2H2(g)+ O2(g)→ 2H2O (l), is interpreted as follows – two moles of hydrogen react with one mole of oxygen to yield two moles of water. In other words, combining 2 grams of hydrogen with 16 grams of oxygen will yield 2 moles of water weighing 18 gm.

Check Point
- One or more chemical substances, known as ______, chemically react to produce one or more ______.
- “Energy can neither be created nor destroyed as a result of a chemical reaction”. This is the law of _________.
- The number of _______ of each element in a chemical equation must remain the same on either side of that equation.
- We make use of ______ to correctly balance chemical equations.
- Iron (Fe) combines with oxygen (O2) to form 2 units of iron oxide Fe2O3. Balance this equation: Fe (s) + O2(g) →Fe2O3(s)
Answer Key
- Reactants, Products
- Conservation of Energy
- Atoms
- Coefficients
- 4Fe (s) + 3O2(g) → 2Fe2O3(s)
What is a chemical reaction?
A chemical reaction is a process where one or more substances undergo a chemical change, resulting in the formation of new substances with different properties.
What are the essential components of a chemical reaction?
The essential components of a chemical reaction are reactants (the substances that undergo the change) and products (the new substances formed). Additionally, energy may be involved in the form of heat or light.
What is a balanced chemical equation?
A balanced chemical equation shows the relative amounts of reactants and products in a chemical reaction. It obeys the law of conservation of mass, meaning that the number of atoms of each element is the same on both sides of the equation.
What is meant by exothermic and endothermic reactions?
An exothermic reaction releases energy in the form of heat or light, resulting in a temperature increase in the surroundings. In contrast, an endothermic reaction absorbs energy from the surroundings, causing a temperature decrease.
What is the difference between a physical change and a chemical change?
In a physical change, the substances involved undergo a change in state or form without altering their chemical composition. In a chemical change, the substances undergo a chemical reaction, resulting in the formation of new substances with different chemical properties.
What are the reactants and products of photosynthesis?
The reactants of photosynthesis are carbon dioxide (CO2) and water (H2O). The products of photosynthesis are glucose (C6H12O6) and oxygen (O2). In addition to glucose and oxygen, photosynthesis also produces water vapor (H2O) as a byproduct.
Learn more about Basics of Chemical Reactions and other important topics with 8th Grade Science Tutoring at eTutorWorld. Our expert science tutors break down the topics through interactive one-to-one sessions. We also offer the advantage of customized lesson plans, flexible schedules and convenience of learning from home.
Schedule a Free session to clear worksheet doubts
No credit card required, no obligation to purchase.
Just schedule a FREE Sessions to meet a tutor and get help on any topic you want!
Pricing for Online Tutoring
| Tutoring Package | Validity | Grade (1-12), College |
|---|---|---|
| 5 sessions | 1 Month | $139 |
| 1 session | 1 Month | $28 |
| 10 sessions | 3 months | $269 |
| 15 sessions | 3 months | $399 |
| 20 sessions | 4 months | $499 |
| 50 sessions | 6 months | $1189 |
| 100 sessions | 12 months | $2249 |
8th Grade Free Worksheets
- The Universe
- Heredity
- Evolutionary Theory
- Structure of the atom
- Ethical Practices
- Unveiling the mystery behind the physical universe
- Components of the universe
- Celestial phenomena
- The tilt of Earth’s axis
- The causes of high and low tides
- Earth Systems
- Rocks and Fossils
- Weather and Climate
- Basics of chemical reactions
- Types of Chemical reactions – Endothermic, exothermic, oxidation, reduction reactions
- Catalysts and enzymes
- Compounds and mixtures
- Acids, Bases and pH Indicators
Images Credit:
https://images.slideplayer.com/26/8750743/slides/slide_5.jpg
IN THE NEWS

Our mission is to provide high quality online tutoring services, using state of the art Internet technology, to school students worldwide.
Online test prep and practice
SCAT
SSAT
ISEE
PSAT
SAT
ACT
AP Exam
Science Tutoring
Physics Tutoring
Chemistry Tutoring
Biology Tutoring
Math Tutoring
Pre-Algebra Tutoring
Algebra Tutoring
Pre Calculus Tutoring
Calculus Tutoring
Geometry Tutoring
Trigonometry Tutoring
Statistics Tutoring
Quick links
Free Worksheets
Fact sheet
Sales Partner Opportunities
Parents
Passive Fundraising
Virtual Fundraising
Our Expert Tutors
Safe and Secure Tutoring
Interactive Online Tutoring
After School Tutoring
Elementary School Tutoring
Middle School Tutoring
High School Tutoring
Home Work Help
Math Tutors New York City
Press
©2022 eTutorWorld Terms of use Privacy Policy Site by Little Red Bird
©2022 eTutorWorld
Terms of use
Privacy Policy
Site by Little Red Bird






